Cancer Data Science Pulse
Your Guide to the 2023 NIH Data Management and Sharing Policy
Do you know how the 2023 NIH Data Management and Sharing Policy will impact your work? If you are an NCI-funded investigator, this blog is for you. We cover the key policy distinctions, implementation changes, and resources to help you navigate the policy.
In this blog
Who should follow the new policy?
Many NIH-funded researchers are familiar with the current NIH Data Sharing Policy (DSP) that was implemented in 2003, which required any NIH-funded research (i.e., basic, clinical, surveys, etc.) requesting more than $500,000 direct cost per year to plan how to share data with the wider community. The soon-to-be-implemented 2023 Data Management and Sharing (DMS) Policy removes this budget threshold and will expect any NIH-funded researcher to provide a DMS Plan detailing:
- what data will be shared.
- how data will be managed and shared.
- when data will be available.
- where data will be preserved, shared, and for how long.
- what, if any, limitations are in place that prevent data sharing (e.g., prior agreements, a lack of informed consent for human data).
- how compliance with the plan will be monitored by the applicant’s institution.
While the 2023 DMS policy will eventually replace the 2003 policy, it’s possible that some investigators may need to follow the 2003 DSP for a while. To determine which policy expectations to follow, check the date of your grant application.
- Who should follow the 2023 DMS Policy?
- Who should follow the 2003 DSP?
- Awards (e.g., Type 5 noncompeting renewals) made before January 24, 2023
What has changed?
We’ve outlined the main differences between the two policies in the table below.
| Changes | 2003 NIH DSP (Current Policy) | 2023 NIH DMS (New Policy) |
|---|---|---|
| Funding Threshold | ≥ $500,000 direct cost per year | Any funding level that results in the generation of scientific data (excluding training and infrastructure development) View the list of NIH activity codes that are subject to the 2023 DMS Policy. |
| Plans | Include a data sharing plan in research proposals detailing how your data will be shared. | Develop a data management and sharing plan that addresses required elements in detail about how you will share and preserve your scientific data. |
| Data Sharing Timelines | At the time of publication | ASAP, no later than the time of publication, or by the end of the performance period (whichever is sooner) |
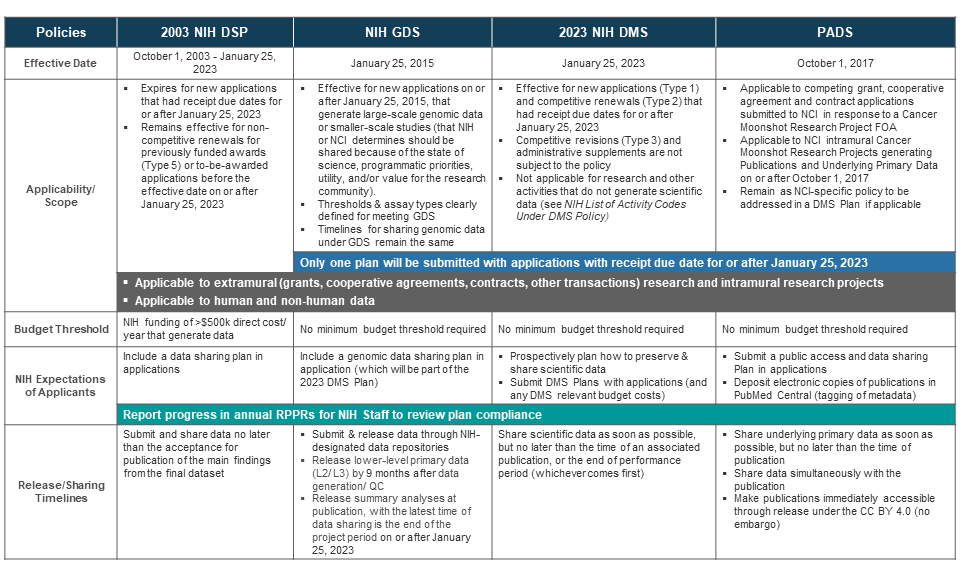
The 2023 DMS Policy also aligns with other data sharing policies you may need to follow. This table compares the expectations for the 2003 NIH DSP, 2023 NIH DMS, NIH Genomic Data Sharing Policy (GDS), and Cancer Moonshot Public Access & Data Sharing Policy (PADS).
Administrative supplements and competing revisions (Type 3) do not need a DMS Plan. However, if an awarded supplemental request (including competing revisions) changes the parent award's approved data management and sharing approach, you should update the DMS Plan of the parent award.
What do I need to do?
If your research grant application had a receipt due date for (or after) January 25, 2023, you will need to follow the expectations outlined in the 2023 DMS Policy.
- Plan for and write a data management and sharing plan according to the required plan elements.
- Submit the data management and sharing plan with your funding proposal.
- Revise and update your plan if necessary. Your program officer or funding contact may reach out through the standard Just-in-Time process for additional information.
- Follow your plan. Once NCI funds the proposal, your plan becomes part of the Terms and Conditions of your award.
- Request to update your plan if something will affect how your data is managed or shared. NCI must approve changes to your plan, so contact your program officer to discuss these updates.
- Provide updates on your data sharing progress in your Research Performance Progress Reports (RPPR).
Read additional guidance information based on your funding mechanism.
Where can I get help?
To assist you, we’ve curated the following resources from NIH’s data sharing website and other websites. If you still need help, add your question to the comment section below or reach out to NCI’s Office of Data Sharing. If you are a grantee, you can also contact your program officer for additional information and guidance.
Resource Links
| Following the policy expectations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drafting data sharing plans |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Choosing a repository | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Practicing the fundamentals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Training Webinars
NIH’s Office of Extramural Research hosted a two-part series to review the changes to the policy. Watch the following videos to learn about the policy, what you need to do, and learn details about your use case:
- A Conversation with NIH: Implementing the New 2023 Data Management and Sharing (DMS) Policy, Part 1
- A Conversation with NIH: Diving Deeper into the New 2023 NIH Data Management and Sharing (DMS) Policy, Part 2
Frequently Asked Questions
Q. My work is covered by multiple policies. Do I need to do separate plans for each?
A. No, you don’t. Only one plan will need to be submitted with applications having a receipt due date for (or after) January 25, 2023.
Q. Is this replacing the Genomic Data Sharing (GDS) Policy?
A. No. NCI-funded researchers generating genomic data should still follow the GDS Policy. NIH is harmonizing the GDS expectation with the new 2023 DMS Policy. To learn more about the GDS Policy and when it applies, read this short overview and implementation change.
Q. Do you have specific guidance about cancer data that we need to follow?
A. Make sure to address the recommended Plan elements outlined in the Supplemental Guidance. You should also follow other NCI policies and program-specific expectations if applicable (e.g., NCI Clinical Trial Access Policy). For example, you may have additional data sharing expectations outlined in your funding opportunity announcement (FOA) or as result of other NCI policies (e.g., Cancer Moonshot℠ Public Access and Data Sharing Policy expectations). Visit the data sharing policies page for a short overview of data sharing policies that impact NCI investigators.
Q. I’m a data scientist/bioinformatician generating secondary data. Does this apply to me?
A. Yes. The DMS Policy applies to research that generates scientific data, which can also result from secondary research. However, you will only be expected to write and follow a DMS Plan for the new secondary data generated or derived from the original data set. You may also need to include the data use limitations from the repositories or resources that you used to obtain the primary data. These expectations would be a justifiable reason for limiting sharing under the 2023 DMS Policy.
Q. I'm an early-career cancer researcher who doesn't know where to begin. What do I do?
A. Let’s start you out on our data sharing “how-to” article. You’ll get information on fundamental tips, expectations, and more!
We’ll continue to update this list of FAQs with common questions from the community.
Leave a Reply
Categories
- Data Sharing (66)
- Informatics Tools (42)
- Training (40)
- Precision Medicine (36)
- Data Standards (36)
- Genomics (36)
- Data Commons (34)
- Data Sets (27)
- Machine Learning (25)
- Artificial Intelligence (25)
- Seminar Series (22)
- Leadership Updates (14)
- Imaging (13)
- Policy (10)
- High-Performance Computing (HPC) (9)
- Jobs & Fellowships (7)
- Semantics (6)
- Funding (6)
- Proteomics (5)
- Information Technology (4)
- Awards & Recognition (3)
- Publications (2)
- Request for Information (2)
- Childhood Cancer Data Initiative (1)

GC on January 11, 2023 at 01:44 p.m.