Data Sharing Policy Guidance
Looking for information on sharing NCI-funded cancer research data? This page details the people, policies, requirements, and resources you need to start sharing your data.
How Does NCI Approach Data Sharing, Management, and Access?
If you’re generating, collecting, leveraging, or managing NCI-funded research data, you’ll need to stay up to date on the latest data sharing requirements.
NCI established the Office of Data Sharing (ODS) to help you navigate these requirements. This team is your authoritative source for information on the policies and processes for sharing and using research data.
ODS’ goal is to make complex NCI and NIH data policies easier to understand and to put into practice. ODS offers a broad range of expertise to help guide you through these policies.
In short, when you have a question about how to share your NCI-funded research, ODS is here to help you.
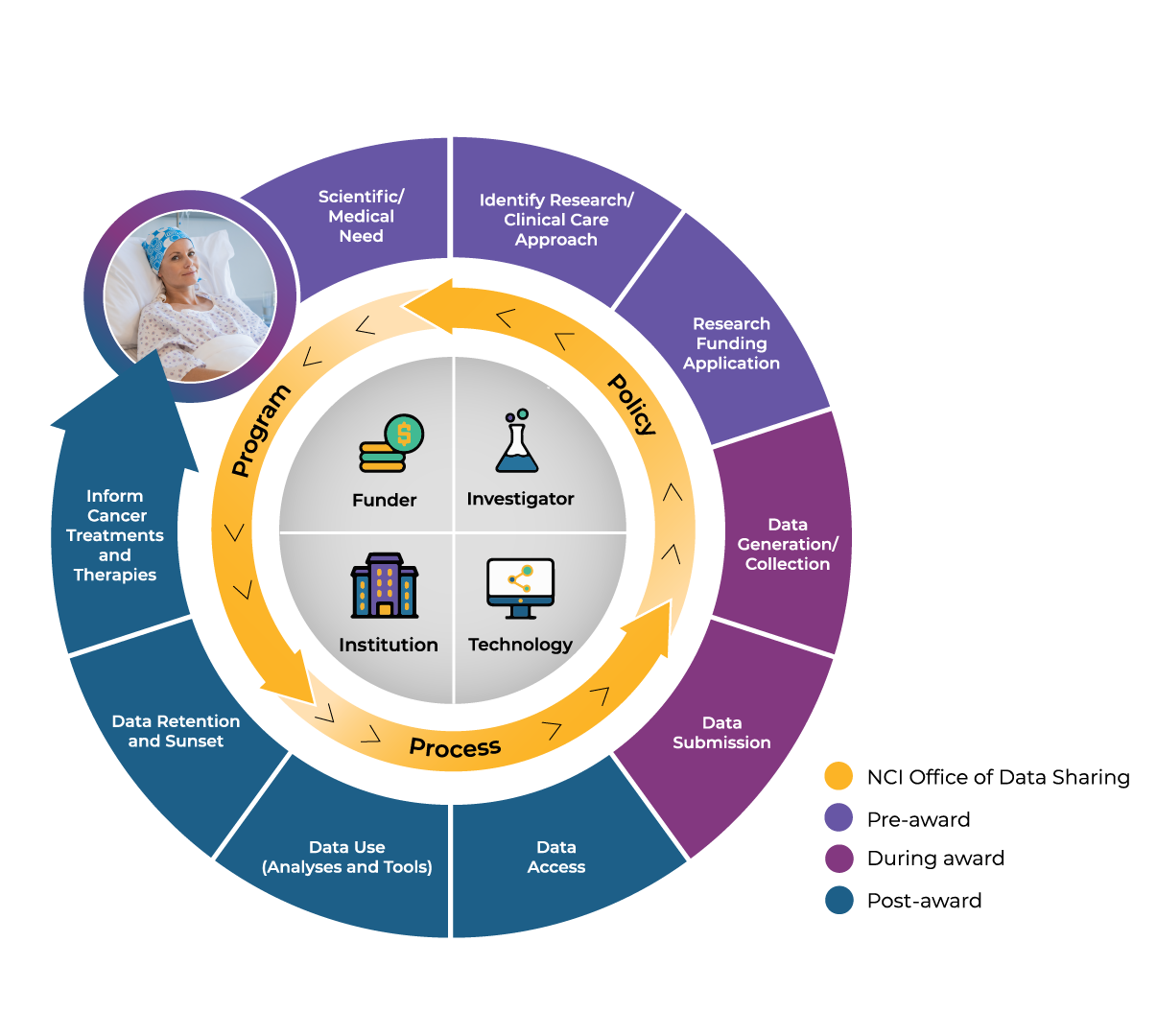
What is NCI’s Overall Data Sharing Process?
NCI’s data sharing approach starts and ends with the patient in mind. So what does this approach look like in its entirety? In this video, ODS Director Dr. Jaime Guidry Auvil will introduce all the stages of the data sharing lifecycle and share how she and ODS can help you navigate the various steps.
As mentioned in the video, ODS supports data sharing in three main ways. They:
- advise on programs that define impactful data and resources,
- implement policies that promote broad access and use of data, and
- offer tools and processes that help ensure data are Findable, Accessible, Interoperable, and Reusable (FAIR).
If you’re working with NCI data and you’re uncertain about what to do or when to do it, we’re happy to help (contact ODS).
What Are Some General Data Sharing Guidelines?
Now that you know more about ODS and the data sharing lifecycle, here are some guiding principles from ODS to help your data be most useful for secondary reuse:
- Designate data for “General Research Use” when you seek informed consent from research participants. This means others can use the data for research purposes only.
- Share your research. When you publish your research, include information on finding and accessing the underlying data (e.g., citations, URLs, and access instructions). Be sure to follow NIH’s rules on making your research open access.
- NCI and NIH data and public access policies outline expectations for making publicly funded information freely, broadly, and rapidly available to the research community. Consider publishing your findings in an open access journal.
- Use existing data standards wherever possible. You can share your data easier when those data adhere to FAIR data standards (i.e., data are Findable, Accessible, Interoperable, and Reusable). Follow FAIR principles and use persistent identifiers (PIDs) for each data set, publication, and biospecimen.
- Adopt common data elements and data file formats, such as those from NCI’s flagship studies (e.g., PADS, The Cancer Imaging Archive, The Childhood Cancer Data Initiative, NCI-MATCH, and NCI’s Surveillance, Epidemiology, and End Results Program).
Which Policies Apply to NCI-Funded Research?
Here’s a sampling of data policies applicable to NCI-funded research. For additional policies on NIH data sharing, visit NIH’s Scientific Data Sharing website.
NIH Data Management and Sharing (DMS) Policy
What is it?
This overarching policy requires investigators to plan how data collected from any research conducted using funds from NIH will be responsibly managed and broadly shared with the research community. Visit the NIH Grants website for implementation details.
How does it apply?
If you’re seeking NIH funding, regardless of the funding amount or mechanism, you’re required to submit a DMS Plan. This includes:
If you submitted a research application between October 1, 2003, and January 24, 2023, you should follow the 2003 Data Sharing Policy.
NIH Genomic Data Sharing (GDS) Policy
What is it?
This policy sets expectations for sharing NIH-funded genomic data broadly and equitably through a supported NIH repository.
How does it apply?
It applies to all NIH-funded research (e.g., grants, contracts, intramural research, regardless of funding level) that generates or reuses genomic data from large-scale human or non-human research.
In addition, NCI may choose to apply this policy to projects that generate small-scale genomic data, such as those that:
- advance the state of the science.
- meet current programmatic priorities.
- add value to the research community.
Cancer MoonshotSM Public Access and Data Sharing (PADS) Policy
What is it?
This NCI policy sets expectations for Cancer Moonshot research projects to better collaborate and share their collective data. Under this policy, applicants submit “Public Access and Data Sharing Plans” that describe their proposed process for making, to the largest extent possible, publications and the underlying primary data immediately and broadly available to the public.
How does it apply?
This policy applies to:
- competing grant and cooperative agreement applications submitted in response to Cancer Moonshot Research Project Funding Opportunity Announcements on or after October 1, 2017.
- proposals for contracts related to Cancer Moonshot Research Projects submitted on or after October 1, 2017.
- NCI intramural Cancer Moonshot Research Projects generating Publications and Underlying Primary Data, submitted on or after October 1, 2017.
Intramural Research Program (IRP) Human Data Sharing (HDS) Policy
What is it?
The goal of this policy is to ensure intramural research program data (either NIH-owned or jointly owned) are available for secondary research purposes, and adhere to the necessary laws, regulations, and policies. In some cases, you may not be able to share all your data because of agreements with outside collaborators (e.g., Cooperative Research and Development Agreements, or “CRADAs,” Clinical Trial Agreements, or other agreements).
How does it apply?
This policy applies to all NIH IRP human data (including the NIH Clinical Center as well as NIH Institutes and Centers).
NIH Clinical Trial Access Policy
What is it?
This policy responds to the need to ensure NCI-supported clinical trial results are publicly available in a timely fashion.
How does it apply?
This policy applies to NCI-funded research grants, cooperative agreements, and/or contracts that support applicable interventional clinical trials. If you’re conducting clinical trials, review this policy to see how you can help make data from clinical trials publicly available.
How Do They Compare?
What Are Some Frequently Asked Questions?
What Resources are Available?
Understanding Policies
- Policy Decision Tool: Use this web application to see which NIH policies might apply to your research.
- Toolkit for DMS Policy: Learn more about the DMS policy by visiting the National Library of Medicine.
- Decision Tree for GDS and 2023 DMS Policy Harmonization: Answer three questions to see if your research must apply the GDS Policy, 2023 DMS Policy, or both.
- OER/OSP Guidance on Informed Consent for Secondary Research with Data and Biospecimens: Use this templated language to write your informed consent documents and maximize data sharing.
NCI Staff: Refer to NCI’s intranet for a video and slides on the DMS Policy, titled, “NCI All Hands DMS Policy Educational Series” and use NCI’s DMS Plan Evaluation Support Tool.
Writing a Plan
- Writing Applicable Activity Codes: Review this list of activity codes to see if you need to write a DMS Plan for your research.
- DMPTool: Sign up to use this free software, which will walk you through creating DMS plans based on NIH templates.
- NIH Federal Demonstration Partnership: Write your DMS plan using one, or preferably both, templates offered by the Federal Demonstration Partnership. After you use these pilot templates, be sure you give feedback! This will help NIH create an NIH-wide plan template in the future.
- Tips for Writing a DMS Plan: Read these quick tips to help you write your plan for NCI-funded or sponsored research.
- Glossary for Broad Data Types for DMS Plans: Review this glossary to get ideas for accurately describing your research data.
- NIH-Supported Scientific Data Repositories: For examples, explore this list of repositories which house various types of data.
Need More Help?
Have general questions about data sharing?
- Submit your question in the comment box below.
- Email NCI’s Office of Data Sharing Team.
- Contact your program officer or intramural program.
Have NCI-specific questions about your NCI-funded genomic data research? Reach out to your program officer, followed by your designated Genomic Program Administrator.
For updates on policy, subscribe to the ODS Newsletter.
For general information on cancer data science and data sharing, subscribe to our weekly Data Science Updates email.