Cancer Data Science Pulse
NCI’s IMMUNOtron—The Making of a Machine Learning (ML) Robotics Tool
If you’re working with ML in the cancer research field, you know two fundamental elements are necessary for success—large amounts of data and a clear physiological target. Researchers from NCI’s Center for Cancer Research, Dr. Grégoire Altan-Bonnet and Mr. Sooraj Achar, have turned to robotics for a solution. In this blog, they describe IMMUNOtron, an ML-and-robotic platform, that’s helping them generate and process greater amounts of data to better understand immune responses.
Armed with this knowledge, researchers and clinicians may soon be able to make more informed decisions about how and when to use immunotherapy for treating cancer.
What does IMMUNOtron do?
IMMUNOtron uses a “high throughput” process that leverages robotics to give us an inside look at immune-cell dynamics, with a particular focus on function—that is, how these cells behave over time.
Initially, we used this robotic platform for measuring cytokines. These tiny signaling molecules help coordinate the immune system’s attack on cancer cells. Cytokines serve as messengers, instructing important immune cells, such as T cells to “call to action” or “stand down.” T cells fight cancer in two ways, acting directly at the tumor site and indirectly by priming other cells to better recognize tumor-specific molecules (called neoantigens) on the tumor itself, which can help kick start a broader immune response.
Neoantigens, with their proximity to the cell surface, serve as critical targets in the development of immunotherapies such as cancer vaccines. T cells recognize neoantigens, docking at specific receptors (i.e., T cell receptor [TCR]) on specific molecules in the cancer cell (called major histocompatibility complex [MHC] molecules). This gives T cells’ killing and inflammatory capacities to stop cancer’s growth and progression.
The wide variety of MHC across a genetically-diverse population of patients makes it difficult to generate a universal TCR to treat every cancer in every patient. However, we can boost the activation of T cells by predicting the tumor cell’s antigen quality. Knowing the quality of targeted antigen molecules on the surface of the cancer cell (i.e., how “foreign” it is, and how well recognized by T cells it can be), gives us a greater chance of successful therapy. The greater the antigenicity of the tumors, the stronger the activation (and killing power) of the T cells against the tumor cells.
Using IMMUNOtron, we can set parameters and model immune responses to tumors before starting treatment to see which patients will benefit the most from immunotherapy. This not only lets clinicians more effectively target therapy but also sharpens tumor targeting to protect healthy tissue and limit toxicity.
What’s the biggest advantage for IMMUNOtron?
The immune response invokes a highly complex “machinery” of cells and molecules. The IMMUNOtron lets us generate enough data to start using more advanced analysis tools, like ML, to help us interpret the nuances underlying that response. With ML, we can easily process greater amounts of data and glean the fundamental and “simple” organizing principles underlying this data. We can look at T cell activation in a way that’s quicker and more efficient than traditional methods, which rely on experiments conducted by hand in a laboratory. We also can map the process over time to see how the immune response changes.
Most importantly, we built our model to be universal, making it quite difficult to “fool.” We can use IMMUNOtron to map changes in antigen quality, regardless of the type of tumors or the state of the responding T cells, and no matter what organism we are studying (mouse or human).
What role does robotics play in your approach?
The gamechanger came in the form of a closed-loop automation. In this loop, the machine can pull out cells growing inside an incubator at different times, test them, and then move that sample into a refrigerator for storage and further analysis. The system does all the work, and we don’t have to interfere at all in the experiment, allowing us to run multiple experiments at once.
We can program the robot to collect time points for each experiment and we can change what action takes place at each time point (e.g., examining cytokines or single cell, etc.). Tracking immune responses relies heavily on such tests to map out various immune cell functions over time. In the past, this meant developing cell cultures, measuring cytokine secretion, tracking immune cell growth and activity, and assessing the effectiveness of the immune response—all by hand. With our system, we’ve been able to automate these key steps.
The full IMMUNOtron robotic platform (see Figure 1) uses three different robots:
- Robot 1: The Tecan robot collects the cytokine samples at different timepoints.
- Robot 2: The Mantis robot tests these samples at different timepoints and at the end of an experiment.
- Robot 3: The Fortessa HTS robot measures the levels of cytokines using flow cytometry.
Finally, we use a python package called “plateypus” to annotate, compile, and process these data.
With this system, we can speed the process of retrieving and testing samples, quickly generating data. Not only do we gain in efficiency, but our results are much more consistent because the process is the same every time. Moreover, robots are good at keeping schedules—there’s no downtime because of nights, weekends, or holidays. The system works reliably every hour of every day.
What’s been the biggest challenge in developing IMMUNOtron?
A lack of data is a key challenge when developing ML models. We built the robotics to specifically tackle this issue. Because the system can run continuously, we can generate high-quality data throughout the day and night with no human intervention. In addition, we’re able to produce a lot of data with very few samples. For example, we can generate more than 30 million, 50-dimensional datapoints from a single mouse. When setting parameters for immune responses using cytokine measurements, we don’t need a lot of cells to make a valuable data set; indeed, we can repeatedly sample a small amount of cell supernatant at different times to assemble a complete timeseries of the experiment.
What’s the future for IMMUNOtron?
At NCI, we’re leveraging technology to revolutionize the way we develop and target immunotherapy.
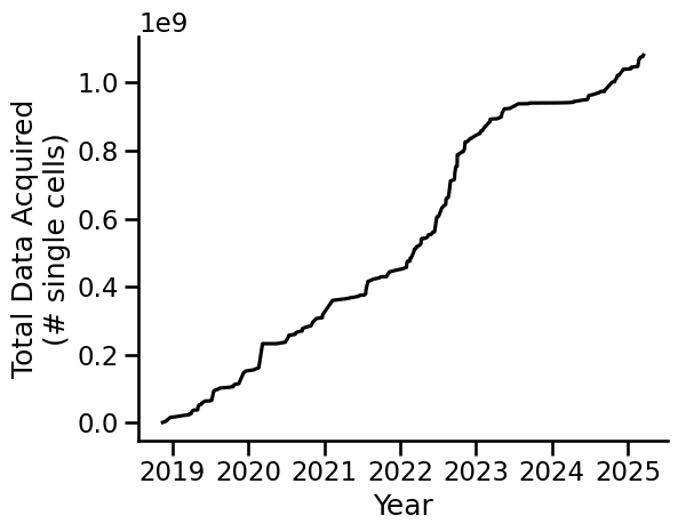
By continuing to improve how we use robotics in data processing, we’re seeing exponential growth in the way we generate and process data. In just a few short years, as we learned how to use the robot more effectively, we’ve been able to dramatically increase the amount of data we’re capturing by starting to focus on measuring single cells in addition to cytokines (see Figure 2).
And we’ve only just begun to tap this technology. IMMUNOtron is becoming a go-to technology to investigate clinical samples (e.g., we’re currently collaborating with Dr. Naomi Taylor’s group from the CCR Pediatric Oncology Branch) and to build better ML models (in our work with Dr. Paul François’s group at the Université de Montréal). Our robotics-and-ML pipeline is giving us greater power for exploring all types of cells and cellular interactions, from cytokines and bulk data to single cell data and beyond.
We’re also in the midst of duplicating this technology at the University of Oxford to test our system in diverse immunological settings. We hope to continue to grow our samples, giving researchers who are involved in clinical manufacturing a way to contribute samples for analysis.
Ultimately, we hope to create an IMMUNOtron hub here at NCI. This central location would allow researchers from all over the world to access immune-response data to aid in developing new therapies and vaccines. Likewise, oncologists would be able to use IMMUNOtron to determine which patients are most likely to benefit from immunotherapy and which treatment will be most effective.
Additional Reading
- Achar SR, Bourassa FX, Rademaker TJ, et al. Universal Antigen Encoding of T Cell Activation From High-Dimensional Cytokine Dynamics. Science 376; 880–884: 2022. (requires paid subscription)
- Kondo T, Bourassa FX, Achar SR, et al. Engineering TCR-Controlled Fuzzy Logic Into CAR T Cells Enhances Therapeutic Specificity. Cell 188; 1–18: 2025. (requires paid subscription)
Categories
- Data Sharing (66)
- Informatics Tools (42)
- Training (40)
- Precision Medicine (36)
- Data Standards (36)
- Genomics (36)
- Data Commons (34)
- Data Sets (27)
- Machine Learning (25)
- Artificial Intelligence (25)
- Seminar Series (22)
- Leadership Updates (14)
- Imaging (13)
- Policy (10)
- High-Performance Computing (HPC) (9)
- Jobs & Fellowships (7)
- Semantics (6)
- Funding (6)
- Proteomics (5)
- Information Technology (4)
- Awards & Recognition (3)
- Publications (2)
- Request for Information (2)
- Childhood Cancer Data Initiative (1)


Leave a Reply