News
FDA's precisionFDA Has Launched "Truth Challenge V2"
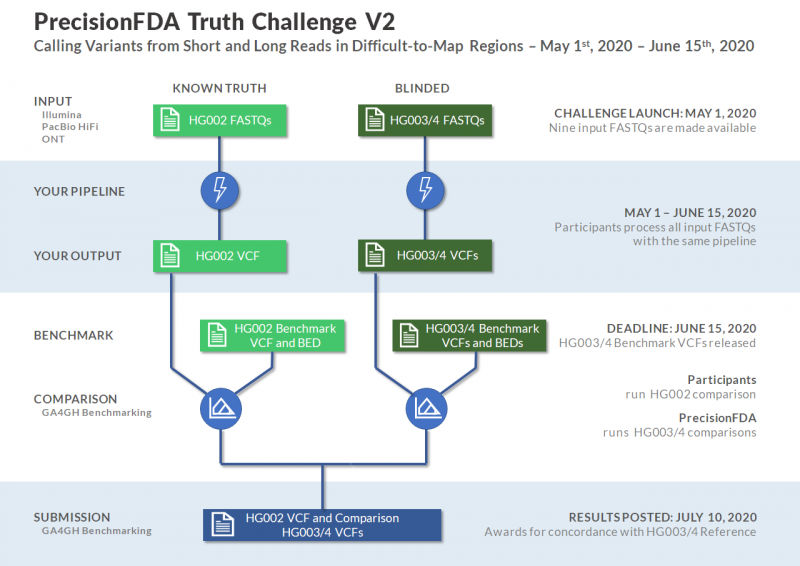
On May 1, 2020, the Food and Drug Administration's (FDA's) precisionFDA launched "Truth Challenge V2: Calling Variants from Short and Long Reads in Difficult-to-Map Regions."
In the context of whole human genome sequencing, software pipelines typically rely on (1) mapping sequencing reads or assemblies to a reference genome, and (2) the subsequent identification of variants. One way of assessing such pipelines' performance is by using well-characterized reference data sets such as Genome in a Bottle’s seven human genome benchmarks. Two of these benchmarks were used in the first precisionFDA Truth Challenge, which assessed variant-calling accuracy in “easier-to-map” regions of the genome. The Genome in a Bottle Consortium, led by the National Institute of Standards and Technology (NIST), has developed expanded benchmarks for HG003 and HG004, the parents of an Ashkenazi trio. Before their release, precisionFDA and NIST are running a new truth challenge focused on variant calling in more challenging genomic regions.
Join this challenge to assess variant-calling pipeline performance on a common frame of reference consisting of difficult-to-map regions, segmental duplications, and the Major Histocompatibility Complex (MHC). Ilumina, PacBio HiFi, and Oxford Nanopore sequencing data sets will be made available for this challenge.
The challenge submission period is open from May 1, 2020, through June 15, 2020. Visit the challenge webpage for more information. Please also note that you are required to register for a precisionFDA user account in order to participate in any challenges.